Your Regulatory Affairs Partner
for Europe and China
Our services
⟶ Expert support during the whole life cycle of your product
We will guide you through the regulatory process of your product and help you to successfully interact with Chinese authorities.
We provide pharmaceutical companies with a qualified Information Officer to coordinate and monitor the organisation’s information management and marketing activities.
Regularly audit your suppliers and service providers, as well as your own internal organization. You will be assisted by QbD’s team of highly qualified and experienced auditors, which ensures an easy and quick procedure.
Where we operate

Europe (EU)
We are based in Vienna, Austria, in the heart of Europe. Our team members come from various corners of the continent - with a broad regulatory expertise and experience with European Health Authorities.

China
The Chinese market is large, diverse and full of opportunities – but it can also be a challenging one. Our China RA experts are both familiar with the Chinese regulations as well as the language and business practices.
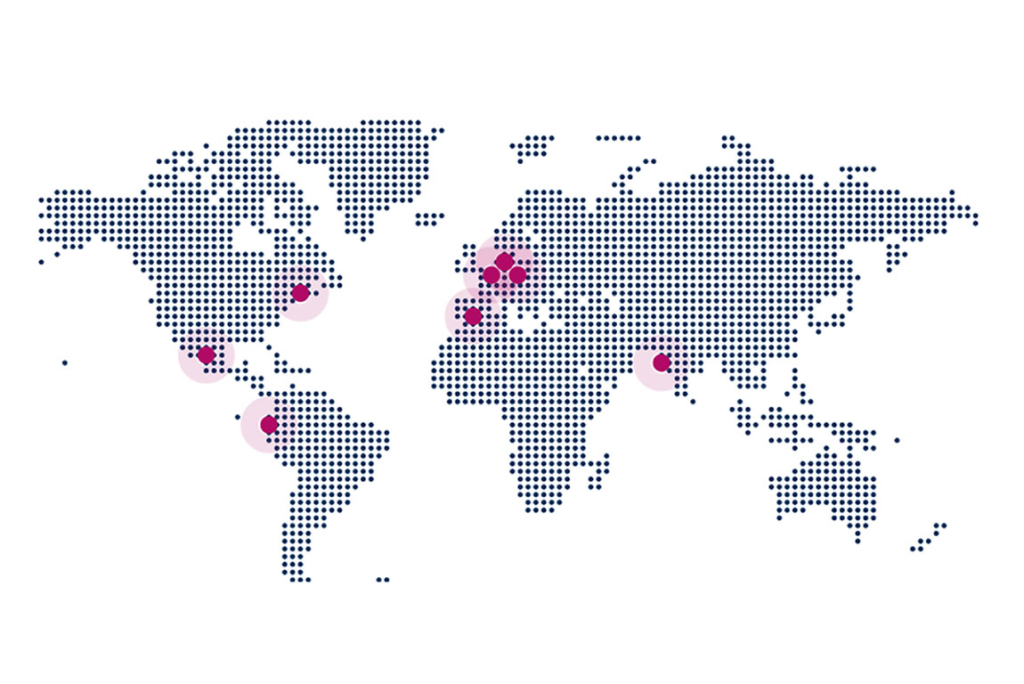
QbD International
QbD Austria is part of the QbD Group, an international organization. With its headquarters in Belgium and offices across the globe (Netherlands, Spain, France, Mexico, Colombia, and many more), QbD is able to execute projects in many places.
Regulatory Topics
Our values
Joy
Partnership
Extra Mile
Getting things done

Joy

Partnership
Alone, you can do something. Together, we can do everything. That’s why QbD continuously invests in partnerships between people, companies and sectors.

Extra Mile

Getting things done
Join our team of highly motivated regulatory experts in Vienna, Austria.

“RAPS Fellow” Salma Michor
Dr. Salma Michor was awarded “RAPS Fellow” at this year’s annual RAPS conference in Montreal, Canada.

EU Regulatory Affairs Master’s Program
Delighted to meet Brian Savoie, Senior Vice President of Regulatory Affairs Professionals Society (RAPS) Education, at the opening of the master’s program “EU Regulatory Affairs” at Donau-Universität Krems.

RAPS Convergence 2023
Dr. Salma Michor opened the RAPS Euro Convergence 2023 in Amsterdam as the new chair of the RAPS EU Board.